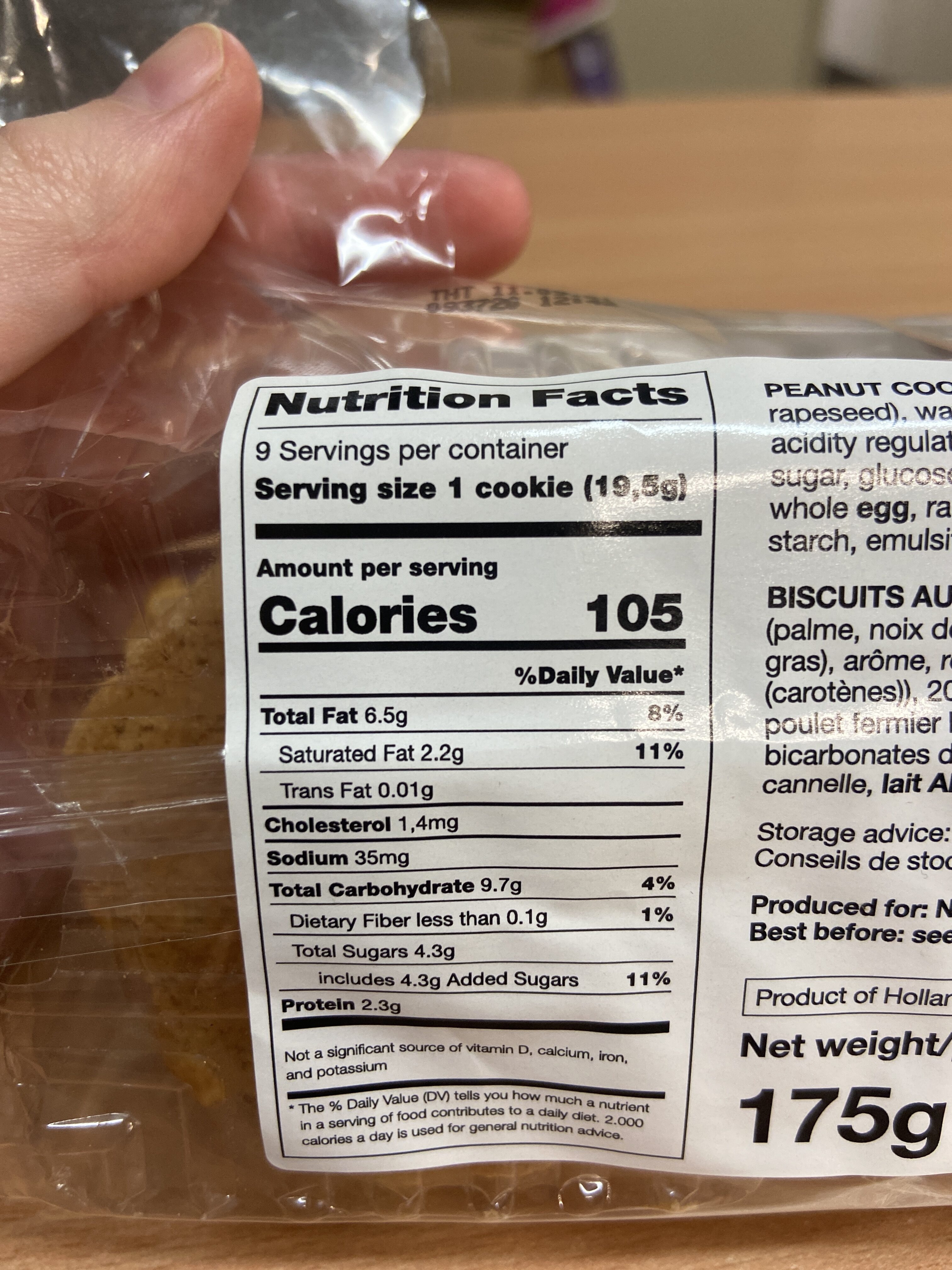
Dutch Bakery - 175 g
This product page is not complete. You can help to complete it by editing it and adding more data from the photos we have, or by taking more photos using the app for Android or iPhone/iPad. Thank you!
×
Barcode: 8717775816485 (EAN / EAN-13)
Quantity: 175 g
Brands: Dutch Bakery
Countries where sold: Australia
Matching with your preferences
Report a problem
Data sources
Product added on by openfoodfacts-contributors
Last edit of product page on by clockwerx.
Product page also edited by roboto-app.
If the data is incomplete or incorrect, you can complete or correct it by editing this page.