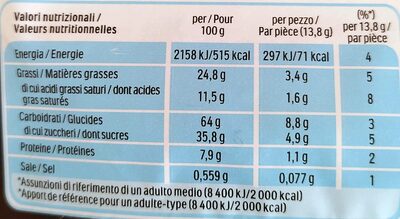
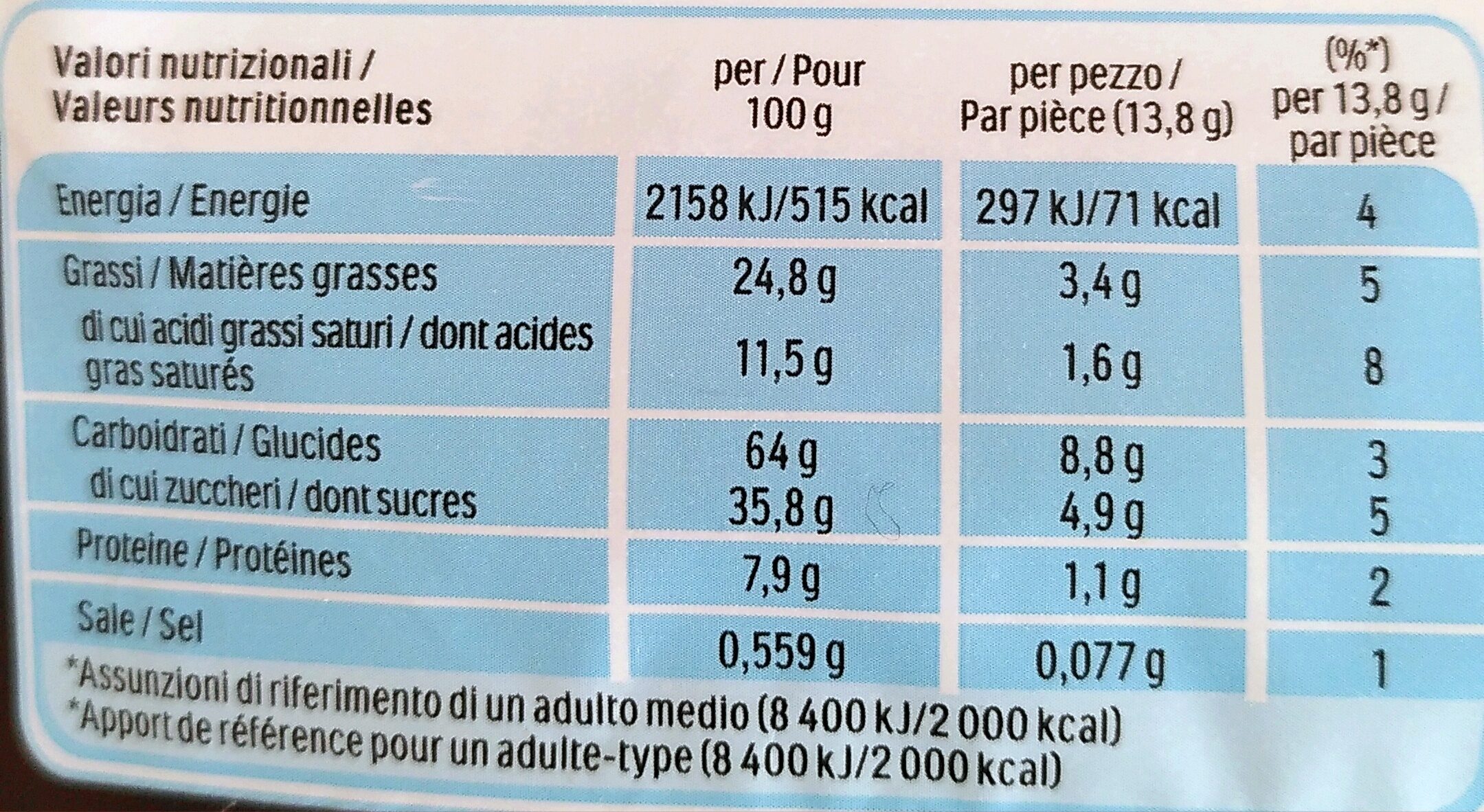
Ferrero- Nutella Biscuits Resealable Bag, 304g (10.7oz) - 304 g
This product page is not complete. You can help to complete it by editing it and adding more data from the photos we have, or by taking more photos using the app for Android or iPhone/iPad. Thank you!
×
Some of the data for this product has been provided directly by the manufacturer FERRERO FRANCE COMMERCIALE.
Barcode: 8000500310427 (EAN / EAN-13)
Common name: Biscuit fourré à la pâte à tartiner aux noisettes et au cacao Nutella®
Quantity: 304 g
Packaging: Plastic, O 7 - Other plastics
Brands: Nutella, Ferrero, Nutella - Ferrero
Categories: Snacks, Sweet snacks, Biscuits and cakes, Biscuits, Filled biscuits
Link to the product page on the official site of the producer: https://www.nutella.com/fr/fr/produits/n...
Stores: Super U Intermarchė carrefour.fr Edeka REWE Carrefour
Countries where sold: France, Germany, Italy, Morocco, Romania, Spain, Switzerland
Matching with your preferences
Other information
Conservation conditions: A conserver au sec et à l'abri de la chaleur. Ne pas mettre au réfrigérateur.
Customer service: FERRERO FRANCE COMMERCIALE - Service Consommateurs, CS 90058 - 76136 MONT SAINT AIGNAN Cedex
Report a problem
Data sources
The manufacturer FERRERO FRANCE COMMERCIALE uses Equadis to automatically transmit data and photos for its products.
Product added on by openfoodfacts-contributors
Last edit of product page on by foodless.
Product page also edited by allergies-app-chakib, antonos65, arlettepipard-gmail-com, arrotino, cgfan749, charlesnepote, chevalstar, citrullus28, cquest, danbernfanck, date-limite-app, desan, driveoff, duhowpi, ecoscore-impact-estimator, elcoco, erleje, feat, ferrero, foodvisor, fpdsurveys, g123k, gmlaa, gourmet, grumpf, heuwerk, inf, insectproductadd, kiliweb, lalucarne, loremily, mairoluin, mathias83dxb, maxtess, monalika9, moncoachigbas, moon-rabbit, mrhalal, org-ferrero-france-commerciale, packbot, prepperapp, pupuce78280, quechoisir, quentinbrd, radek, raphael0202, roland5457, salma124, scanbot, sebleouf, segundo, smoothie-app, stephane, swipe-studio, tacite, telperion87, thaialagata, virginie0031, yuka.U29JTEFmMDlpTXRSc3NJdytnbnM1OUFzNllDdGYzaWFMY000SUE9PQ, yuka.YTRzaExZWWNpdEF1cXNZZnd5UGt4L1Z6bDZhZ1dXeU1Mc002SUE9PQ, yuka.ZEtvL0x2US9vOThKa3NFNjd4Q01xK2d1N1pQMFgyaXJDdHNhSVE9PQ, yuka.ZTc0d1M2UlFsOTR2c00wZSt4UGwvb2x3eksyWFRFZnBEZTB6SUE9PQ, ztoclo.
Last check of product page on by telperion87.