Help us make food transparency the norm!
As a non-profit organization, we depend on your donations to continue informing consumers around the world about what they eat.
The food revolution starts with you!
Pépito Chocolat au lait - LU - 400 G (2 × 200 G)
Pépito Chocolat au lait - LU - 400 G (2 × 200 G)
This product page is not complete. You can help to complete it by editing it and adding more data from the photos we have, or by taking more photos using the app for Android or iPhone/iPad. Thank you!
×
Barcode: 3056440031736 (EAN / EAN-13)
Common name: Biscuit chocolat au lait
Quantity: 400 G (2 × 200 G)
Packaging: Plastic, Bag, Cardboard, fr:Par 2
Brands: LU, Kraft Foods
Categories: Snacks, Sweet snacks, Biscuits and cakes, Biscuits, Chocolate biscuits, Milk chocolate biscuits, Digestives covered with chocolate
Stores: Carrefour
Countries where sold: France
Matching with your preferences
Health
Ingredients
-
26 ingredients
: Farine de blé, chocolat au lait 28% (sucre, pâte de cacao, beurre de cacao, lactose et protéines de lait, lait écrémé en poudre, matière grasse végétale, beurre pâtissier, lactose, émulsifiants (lécithine de soja, E476), arôme), matière grasse végétale, sucre, lactose et protéine de lait, noix de coco, sirop de glucose-fructose, sel, poudre à lever (carbonate acide de sodium, carbonate acide d'ammonium), correcteur d'acidité (orthophosphate monocalcique), arômeAllergens: Gluten, Milk, SoybeansTraces: Eggs, Gluten, Milk, Nuts, Sesame seeds, Soybeans
Food processing
-
Ultra processed foods
Elements that indicate the product is in the 4 - Ultra processed food and drink products group:
- Additive: E322 - Lecithins
- Additive: E476 - Polyglycerol polyricinoleate
- Ingredient: Emulsifier
- Ingredient: Flavouring
- Ingredient: Glucose
- Ingredient: Lactose
- Ingredient: Milk proteins
Food products are classified into 4 groups according to their degree of processing:
- Unprocessed or minimally processed foods
- Processed culinary ingredients
- Processed foods
- Ultra processed foods
The determination of the group is based on the category of the product and on the ingredients it contains.
Additives
-
E322 - Lecithins
Lecithins are natural compounds commonly used in the food industry as emulsifiers and stabilizers.
Extracted from sources like soybeans and eggs, lecithins consist of phospholipids that enhance the mixing of oil and water, ensuring smooth textures in various products like chocolates, dressings, and baked goods.
They do not present any known health risks.
-
E322i - Lecithin
Lecithins are natural compounds commonly used in the food industry as emulsifiers and stabilizers.
Extracted from sources like soybeans and eggs, lecithins consist of phospholipids that enhance the mixing of oil and water, ensuring smooth textures in various products like chocolates, dressings, and baked goods.
They do not present any known health risks.
-
E341 - Calcium phosphates
Calcium phosphate: Calcium phosphate is a family of materials and minerals containing calcium ions -Ca2+- together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. They are white solids of nutritious value.Source: Wikipedia
-
E341i - Monocalcium phosphate
Calcium phosphate: Calcium phosphate is a family of materials and minerals containing calcium ions -Ca2+- together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. They are white solids of nutritious value.Source: Wikipedia
-
E476 - Polyglycerol polyricinoleate
Polyglycerol polyricinoleate: Polyglycerol polyricinoleate -PGPR-, E476, is an emulsifier made from glycerol and fatty acids -usually from castor bean, but also from soybean oil-. In chocolate, compound chocolate and similar coatings, PGPR is mainly used with another substance like lecithin to reduce viscosity. It is used at low levels -below 0.5%-, and works by decreasing the friction between the solid particles -e.g. cacao, sugar, milk- in molten chocolate, reducing the yield stress so that it flows more easily, approaching the behaviour of a Newtonian fluid. It can also be used as an emulsifier in spreads and in salad dressings, or to improve the texture of baked goods. It is made up of a short chain of glycerol molecules connected by ether bonds, with ricinoleic acid side chains connected by ester bonds. PGPR is a yellowish, viscous liquid, and is strongly lipophilic: it is soluble in fats and oils and insoluble in water and ethanol.Source: Wikipedia
-
E500 - Sodium carbonates
Sodium carbonates (E500) are compounds commonly used in food preparation as leavening agents, helping baked goods rise by releasing carbon dioxide when they interact with acids.
Often found in baking soda, they regulate the pH of food, preventing it from becoming too acidic or too alkaline. In the culinary world, sodium carbonates can also enhance the texture and structure of foods, such as noodles, by modifying the gluten network.
Generally recognized as safe, sodium carbonates are non-toxic when consumed in typical amounts found in food.
-
E500ii - Sodium hydrogen carbonate
Sodium hydrogen carbonate, also known as E500ii, is a food additive commonly used as a leavening agent.
When added to recipes, it releases carbon dioxide gas upon exposure to heat or acids, causing dough to rise and resulting in a light, fluffy texture in baked goods.
It is generally recognized as safe (GRAS) by regulatory authorities when used in appropriate quantities and poses no significant health risks when consumed in typical food applications.
-
E503 - Ammonium carbonates
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Source: Wikipedia
-
E503ii - Ammonium hydrogen carbonate
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Source: Wikipedia
Ingredients analysis
-
May contain palm oil
Ingredients that may contain palm oil: Vegetable fat, Butterfat, Vegetable fat
-
Non-vegan
Non-vegan ingredients: Milk chocolate, Lactose and milk proteins, Skimmed milk powder, Butterfat, Lactose, Lactose and milk proteins
-
Maybe vegetarian
Ingredients that may not be vegetarian: Flavouring, Flavouring
-
Details of the analysis of the ingredients
: Farine de blé, chocolat au lait 28% (sucre, pâte de cacao, beurre de cacao, lactose et protéines de lait, lait écrémé en poudre, matière grasse végétale, beurre pâtissier, lactose, émulsifiants (lécithine de soja, e476), arôme), matière grasse végétale, sucre, lactose et protéine de lait, noix de coco, sirop de glucose-fructose, sel, poudre à lever (carbonate acide de sodium, carbonate acide d'ammonium), correcteur d'acidité (orthophosphate monocalcique), arôme- Farine de blé -> en:wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 28 - percent_max: 72
- chocolat au lait -> en:milk-chocolate - vegan: no - vegetarian: yes - ciqual_food_code: 31004 - percent_min: 28 - percent: 28 - percent_max: 28
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 2.8 - percent_max: 28
- pâte de cacao -> en:cocoa-paste - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 16030 - percent_min: 0 - percent_max: 14
- beurre de cacao -> en:cocoa-butter - vegan: yes - vegetarian: yes - ciqual_food_code: 16030 - percent_min: 0 - percent_max: 9.33333333333333
- lactose et protéines de lait -> en:lactose-and-milk-proteins - vegan: no - vegetarian: yes - percent_min: 0 - percent_max: 7
- lait écrémé en poudre -> en:skimmed-milk-powder - vegan: no - vegetarian: yes - ciqual_food_code: 19054 - percent_min: 0 - percent_max: 5.6
- matière grasse végétale -> en:vegetable-fat - vegan: yes - vegetarian: yes - from_palm_oil: maybe - percent_min: 0 - percent_max: 4.66666666666667
- beurre pâtissier -> en:butterfat - vegan: no - vegetarian: yes - from_palm_oil: maybe - ciqual_food_code: 16401 - percent_min: 0 - percent_max: 4
- lactose -> en:lactose - vegan: no - vegetarian: yes - percent_min: 0 - percent_max: 3.5
- émulsifiants -> en:emulsifier - percent_min: 0 - percent_max: 3.11111111111111
- lécithine de soja -> en:soya-lecithin - vegan: yes - vegetarian: yes - ciqual_food_code: 42200 - percent_min: 0 - percent_max: 3.11111111111111
- e476 -> en:e476 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 1.55555555555556
- arôme -> en:flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 2.8
- matière grasse végétale -> en:vegetable-fat - vegan: yes - vegetarian: yes - from_palm_oil: maybe - percent_min: 0 - percent_max: 28
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 0 - percent_max: 22
- lactose et protéine de lait -> en:lactose-and-milk-proteins - vegan: no - vegetarian: yes - percent_min: 0 - percent_max: 14.6666666666667
- noix de coco -> en:coconut - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 15006 - percent_min: 0 - percent_max: 11
- sirop de glucose-fructose -> en:glucose-fructose-syrup - vegan: yes - vegetarian: yes - ciqual_food_code: 31077 - percent_min: 0 - percent_max: 8.8
- sel -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058 - percent_min: 0 - percent_max: 0.7366
- poudre à lever -> en:raising-agent - percent_min: 0 - percent_max: 0.7366
- carbonate acide de sodium -> en:e500ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.7366
- carbonate acide d'ammonium -> en:e503ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.3683
- correcteur d'acidité -> en:acidity-regulator - percent_min: 0 - percent_max: 0.7366
- orthophosphate monocalcique -> en:e341 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.7366
- arôme -> en:flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 0.7366
Nutrition
-
Bad nutritional quality
⚠ ️Warning: the amount of fruits, vegetables and nuts is not specified on the label, it was estimated from the list of ingredients: 1This product is not considered a beverage for the calculation of the Nutri-Score.
Positive points: 3
- Proteins: 4 / 5 (value: 6.8, rounded value: 6.8)
- Fiber: 3 / 5 (value: 3, rounded value: 3)
- Fruits, vegetables, nuts, and colza/walnut/olive oils: 0 / 5 (value: 1.375, rounded value: 1.4)
Negative points: 25
- Energy: 6 / 10 (value: 2050, rounded value: 2050)
- Sugars: 6 / 10 (value: 30, rounded value: 30)
- Saturated fat: 10 / 10 (value: 12, rounded value: 12)
- Sodium: 3 / 10 (value: 294.64, rounded value: 294.6)
The points for proteins are not counted because the negative points are greater or equal to 11.
Nutritional score: (25 - 3)
Nutri-Score:
-
Nutrient levels
-
Fat in high quantity (22%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
Saturated fat in high quantity (12%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
Sugars in high quantity (30%)
What you need to know- A high consumption of sugar can cause weight gain and tooth decay. It also augments the risk of type 2 diabetes and cardio-vascular diseases.
Recommendation: Limit the consumption of sugar and sugary drinks- Sugary drinks (such as sodas, fruit beverages, and fruit juices and nectars) should be limited as much as possible (no more than 1 glass a day).
- Choose products with lower sugar content and reduce the consumption of products with added sugars.
-
Salt in moderate quantity (0.737%)
What you need to know- A high consumption of salt (or sodium) can cause raised blood pressure, which can increase the risk of heart disease and stroke.
- Many people who have high blood pressure do not know it, as there are often no symptoms.
- Most people consume too much salt (on average 9 to 12 grams per day), around twice the recommended maximum level of intake.
Recommendation: Limit the consumption of salt and salted food- Reduce the quantity of salt used when cooking, and don't salt again at the table.
- Limit the consumption of salty snacks and choose products with lower salt content.
-
-
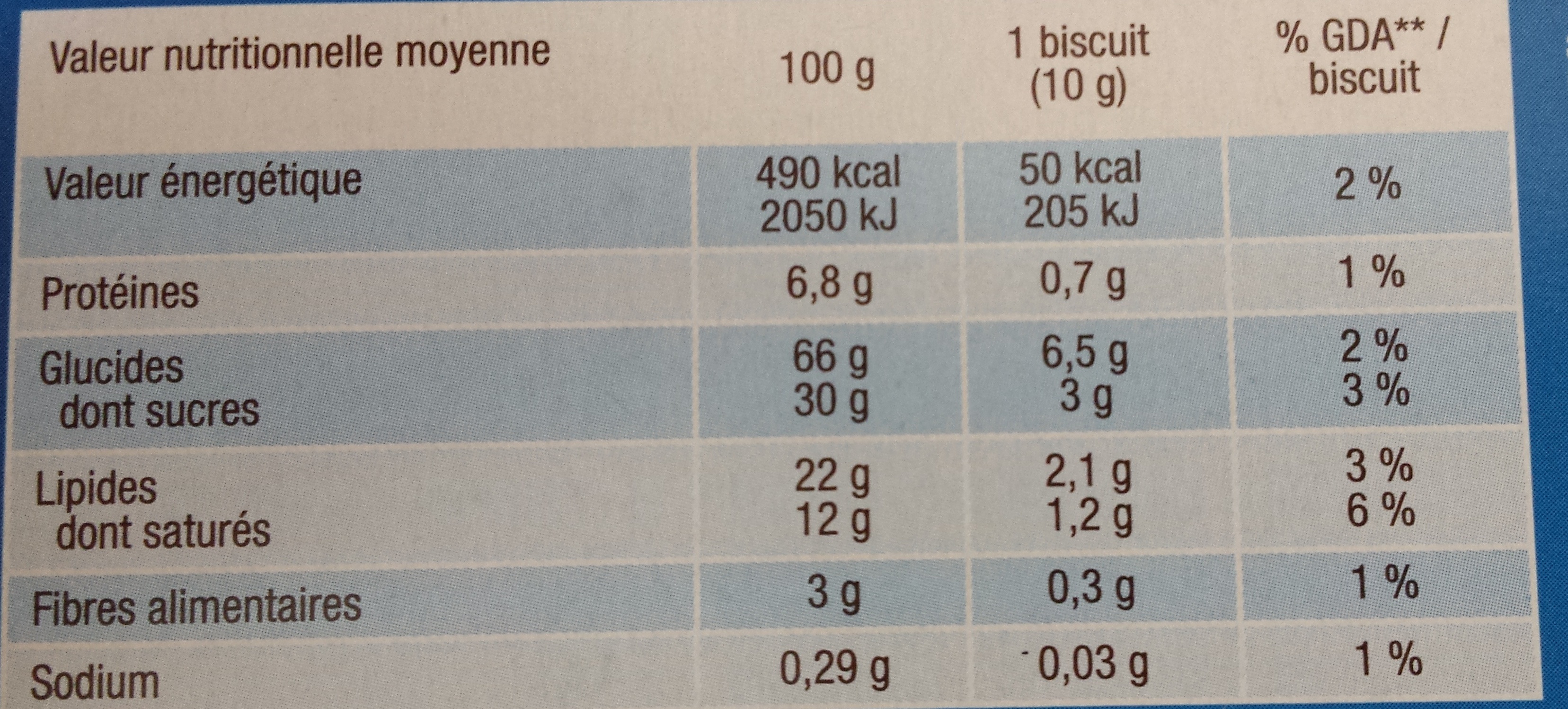
Nutrition facts
Nutrition facts As sold
for 100 g / 100 mlAs sold
per serving (10 g)Compared to: Digestives covered with chocolate Energy 2,050 kj
(490 kcal)205 kj
(49 kcal)-1% Fat 22 g 2.2 g -6% Saturated fat 12 g 1.2 g +4% Carbohydrates 66 g 6.6 g +6% Sugars 30 g 3 g +11% Fiber 3 g 0.3 g -20% Proteins 6.8 g 0.68 g -2% Salt 0.737 g 0.074 g -3% Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 1.375 % 1.375 %
Environment
-
Eco-Score C - Moderate environmental impact
⚠ ️Select a country in order to include the full impact of transportation.The Eco-Score is an experimental score that summarizes the environmental impacts of food products.→ The Eco-Score was initially developped for France and it is being extended to other European countries. The Eco-Score formula is subject to change as it is regularly improved to make it more precise and better suited to each country.Life cycle analysis
-
Average impact of products of the same category: C (Score: 56/100)
Category: Biscuit (cookie), with chocolate, prepacked
Category: Biscuit (cookie), with chocolate, prepacked
- PEF environmental score: 0.47 (the lower the score, the lower the impact)
- including impact on climate change: 5.92 kg CO2 eq/kg of product
Stage Impact Agriculture
63.6 %Processing
29.8 %Packaging
2.3 %Transportation
3.2 %Distribution
1.0 %Consumption
0.0 %
Bonuses and maluses
-
Missing origins of ingredients information
Malus: -5
⚠ ️ The origins of the ingredients of this product are not indicated.
If they are indicated on the packaging, you can modify the product sheet and add them.
If you are the manufacturer of this product, you can send us the information with our free platform for producers.
-
Packaging with a medium impact
Malus: -11
Shape Material Recycling Impact Bag Plastic High Unknown Cardboard Low ⚠ ️ The information about the packaging of this product is not sufficiently precise (exact shapes and materials of all components of the packaging).⚠ ️ For a more precise calculation of the Eco-Score, you can modify the product page and add them.
If you are the manufacturer of this product, you can send us the information with our free platform for producers.
Eco-Score for this product
-
Impact for this product: C (Score: 40/100)
Product: Pépito Chocolat au lait - LU - 400 G (2 × 200 G)
Life cycle analysis score: 56
Sum of bonuses and maluses: -16
Final score: 40/100
-
Carbon footprint
-
Equal to driving 3.1 km in a petrol car
592 g CO² per 100g of product
The carbon emission figure comes from ADEME's Agribalyse database, for the category: Biscuit (cookie), with chocolate, prepacked (Source: ADEME Agribalyse Database)
Stage Impact Agriculture
52.9 %Processing
42.0 %Packaging
1.9 %Transportation
2.9 %Distribution
0.3 %Consumption
0.0 %
Packaging
-
Packaging with a medium impact
-
Packaging parts
Bag (Plastic)
(Cardboard)
-
Packaging materials
Material % Packaging weight Packaging weight per 100 g of product Paper or cardboard Plastic Total
-
Transportation
-
Origins of ingredients
Missing origins of ingredients information
⚠ ️ The origins of the ingredients of this product are not indicated.
If they are indicated on the packaging, you can modify the product sheet and add them.
If you are the manufacturer of this product, you can send us the information with our free platform for producers.Add the origins of ingredients for this product Add the origins of ingredients for this product
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Data sources
Product added on by jitrixis
Last edit of product page on by packbot.
Product page also edited by moon-rabbit, pateuch53.