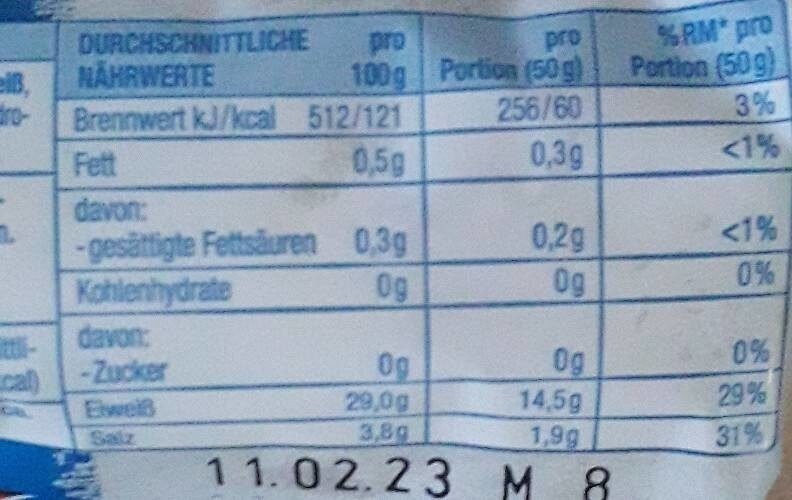
Harzer Käse - Gut&Günstig - 200g
This product page is not complete. You can help to complete it by editing it and adding more data from the photos we have, or by taking more photos using the app for Android or iPhone/iPad. Thank you!
×
Barcode: 4311501677230 (EAN / EAN-13)
Quantity: 200g
Brands: Gut&Günstig, Edeka
Categories: Dairies, Fermented foods, Fermented milk products, Cheeses, Desserts, Dairy desserts, Quarks, Soured milk quark
Labels, certifications, awards:
Vegetarian, No GMOs, Ohne Gentechnik
Origin of ingredients: Germany
Manufacturing or processing places: Deutschland
Traceability code: DE ST 225 EG
Stores: Edeka
Countries where sold: Germany
Matching with your preferences
Environment
Carbon footprint
Packaging
Transportation
Report a problem
Data sources
Product added on by tenlight
Last edit of product page on by roboto-app.
Product page also edited by ecoscore-impact-estimator, kiliweb, openfoodfacts-contributors, yuka.sY2b0xO6T85zoF3NwEKvlhEbYdDchzPmOj_TiFOwyd6lDpfnT81j-ZLjH6s.